Closed-loop spray dryers are suited for organic solvent feed since it uses nitrogen as drying gas to prevent explosion. The main application of such equipment is to spray dry poorly-soluble APIs, which do not dissolve in water but do so in organic solvents.
Please refer to our open-loop spray drying for particle engineeringfa-caret-right
Solid Dispersion
Solid dispersions, which is a technology for improving the absorption of poorly soluble drugs, can be easily manufactured with an organic solvent-based spray dry processing. Due to a remarkable increase of poorly soluble NCEs , an organic solvent spray dryering is attracting attention as a method to improve solubility.
Fuji Chemical Co., Ltd. provides various solutions for solubility enhancement using our closed-loop spray dryers.
What is solid dispersion?
Solid dispersion is defined as “dispersion of one or more active pharmaceutical ingredients in an inert carrier or matrix at solid state” (W. L. Chiou, S. Riegelman: J. Pharm. Sci., 60, 1281, 1971. ) Methods to produce solid dispersion include melt method, solvent method, melting solvent method, and hot-melt extrusion method.
An amorphous solid dispersion of a poorly soluble drug is known to improve the solubility and bioavailability, and the difference in blood concentration due to the presence of food in the stomach is eliminated.
By using a closed-loop spray dryer, it is possible to easily produce a solid dispersion by simply dissolving a drug and a polymer carrier in an organic solvent.
Dissolution Enhancement
In recent years, there have been an increasing number of cases in which new drug candidate compounds have to be abandoned due to their poor solubility. To meet this demand, we have track record of the production of solid dispersions, which is an effective technology for improving the dissolution of poorly soluble drugs.
We have multiple spray dryers that can be used for each development stage (early to commercial.)
- Formulation Study
- Toxicity study sample manufacturing
- Manufacturing of investigational drug
- Commercial production
Spray dried dispersion manufactured at Fuji has already been used in pharmaceutical products around the world, including Japan, the United States, and Europe. Fuji has also been inspected by authorities around the world. We can also manufacture tablets and capsules downstream as well as the packaging of tablets and capsules.
Please contact us from the below link:
Solid dispersion manufacturing methods
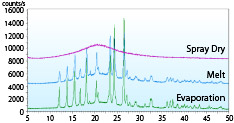 The XRD (X-ray diffraction method) pattern of a solid dispersion of acetaminophen (acetaminophen: PVP = 1: 0.35) produced by spray-drying, hot-melting, and evaporation methods is shown.
The XRD (X-ray diffraction method) pattern of a solid dispersion of acetaminophen (acetaminophen: PVP = 1: 0.35) produced by spray-drying, hot-melting, and evaporation methods is shown.
Only spray-dried products show a halo pattern, indicating that they can be amorphized with less carrier than other manufacturing methods.
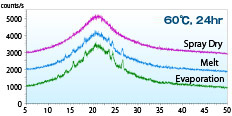 The XRD pattern of a solid dispersion of acetaminophen: PVP = 1: 1 produced by spray-drying, hot-melting, and evaporation methods after being stored at 60 ° C for 24 hours was shown.
The XRD pattern of a solid dispersion of acetaminophen: PVP = 1: 1 produced by spray-drying, hot-melting, and evaporation methods after being stored at 60 ° C for 24 hours was shown.Immediately after production, they all showed a halo pattern. However, only spray-dried products maintained the halo pattern after 24 hours, indicating better stability than other manufacturing methods.
Dissolution Improvement
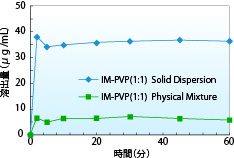 The dissolution test results of a solid dispersion consisting of indomethacin and PVP are shown. The dissolution rate of spray-dried solid dispersion of indomethacin and PVP is approximately 6 times of that of the physical mixture. Maintaining a supersaturated state for a long time leads to improved drug absorption.
The dissolution test results of a solid dispersion consisting of indomethacin and PVP are shown. The dissolution rate of spray-dried solid dispersion of indomethacin and PVP is approximately 6 times of that of the physical mixture. Maintaining a supersaturated state for a long time leads to improved drug absorption.
ACU
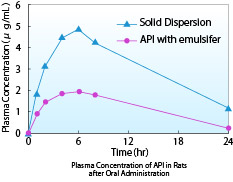 The rat blood kinetics results of the API and its solid dispersion were graphed. The solid dispersion showed a higher AUC than the formulation with milled API and solubilizer.
The rat blood kinetics results of the API and its solid dispersion were graphed. The solid dispersion showed a higher AUC than the formulation with milled API and solubilizer.
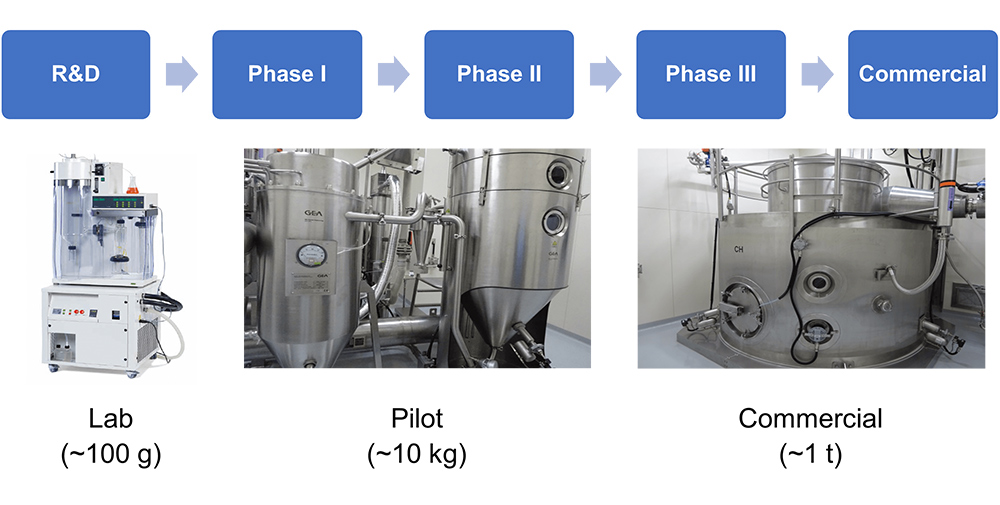
Scale up
Fuji Chemical Co., Ltd. owns spray dryers for a lab scale study to commercial production.