Fujicalin® is a dibasic calcium phosphate anhydrous designed to function as a direct compression excipient.
It has exceptional flow and compression characteristics while maintaining the ability for rapid disintegration.
The key to Fujicalin®’s superior performance is the highly specialized and proprietary manufacturing process that yields a unique primary particle.
The patented manufacturing process yields porous spheres with high specific surface area. Fujicalin® is totally synthetic and ideally suited to direct compression formulations, especially involving difficult-to-compress materials like oily actives.
It can also be used to assist flow, reduce tablet weight variation and improve content uniformity.
Fujicalin®’s compressibility facilitates the design of smaller tablets. It can also be used as a partial or total replacement for microcrystalline cellulose.
General Properties
| Properties | Fujicalin® | |
| Appearance | White Crystalline Powder | |
| Mean particle size(µm) | 120 | |
| Bulk density(g/ml) | Loose | 0.46 |
| Tapped | 0.54 | |
| Compressibility index | 15.1 | |
| Angle of repose(°) | 29.5 | |
| Angle of spatula(°) | 33.3 | |
| Carr value* | 86.5 | |
| BET surface area(m²/g) | 40 | |
| Oil adsorption capacity(ml/g) | 1.1 | |
| Water adsorption capacity(ml/g) | 1.2 | |
| Loss on drying | 0.5 | |
| Flowability | Excellent | |
| Watter activity | 0.11 | |
*Carr value calculated adding relevant index points. Higher value indicates better powder flor properties
(Ref-Carr,R.L., Chem. Eng., 1965; 72(3), 163-168)
Unique attributes of Fujicalin®
Anhydrous Dibasic Calcium Phosphate is generally used as an excipient with low reactivity due to its neutral pH in the aqueous suspension. It also as a high bulk density and is not moisture sensitive as other excipients.
However, compressibility of conventional anhydrous calcium hydrogen phosphate are not sufficient for the direct compression.
Fujicalin® has a spherical granule formed by spray-drying. It is also synthesized by a unique crystal growth control method. Fujicalin® has a crystalline structure of anhydrous calcium hydrogen phosphate, but the X-ray analysis pattern is broad due to the unique synthesis process. The primary particles are fine and plate-like.
With spray drying, plate-like crystals form a card house structure and resulting in granules with a large porosity. This structure produces the characteristics of Fujicalin (compressibility and oil absorption) that a conventional anhydrous dibasic calcium phosphate does not have, and is particularly suitable for direct compression.
Comparison of physical properties with conventional DCPA’s and DCPD
| Property | DCPA ( dibasic calcium phosphate, anhydrous ) | DCPD (dibasic calcium phosphate, dihydrate) |
|
| Fujicalin | Conventional DCPA | ||
| Average particle size* | 120μm | 45μm | 127μm |
| Bulk Density(Loose) | 0.46g/mL | 0.77g/mL | 0.83g/mL |
| Bulk Density(Tapped) | 0.54mg/mL | 1.11g/mL | 0.91g/mL |
| Angle of Repose | 30° | 42° | 35° |
| BET Surface area | 40m²/g | 0.7m²/g | 0.57m²/g |
| Oil Adsorption Capacity | 1.1mL/g | 0.4mL/g | 0.2mL/g |
| Water Adsorption | 1.2mL/g | 0.5mL/g | 0.2mL/g |
*Sieving Method
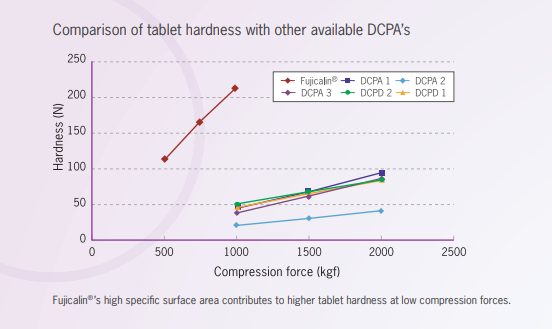
Comparison of tablet hardness with conventional DCPA’s and DCPD
DCPA:dibasic calcium phosphate, anhydrous
DCPD:dibasic calcium phosphate, dihydrate
Summary
- Fujicalin® retains porosity at high compression forces and exhibits low friability across broad compression range
- High porosity is maintained after compression
- Highly flowable and promotes content uniformity
- It can improve disintegration by triggering capillary action. With a right disintegrant, it can create fast disintegration tablets.
- Fujicalin®’s smooth and spherical granules are less abrasive on tableting machines leading to trouble free operations.
Application of Fujicalin®
Fujicalin is used for these applications utilizing the aforementioned attributes
- Fujicalin® makes sufficiently hard tablets at low compression forces, and it has a very low water content in addition. It is an ideal excipient for manufacturing tablets of probiotic preparations
- disintegration tablets using Fujicalin have already been launched commercially. Fujicalin has ability to promote disintegration.
- Fujicalin® is ideal as a carrier for Self-Emulsifying Drug Delivery System (SEDDS) and solid dispersion including Hot Melt Extrusion (HME).
- There have been roller compaction applications utilizing Fujicalin and micro crystalline cellulose