Neusilin® possesses excellent adsorption capacity for its extremely large specific surface area and porous reticulate structure. It can adsorb up to 330% of its own weight and maintains its powdery state.
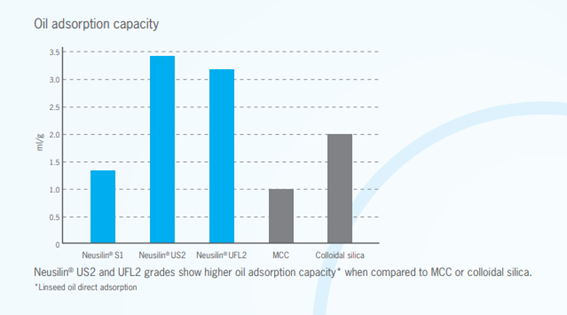
Neusilin’s Oil Adsorption Capacity
Powderization of Silicon Oil with Neusilin® US2 (Video)
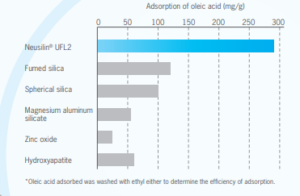
Neusilin® as an adsorption carrier
Liquids such as oils and extracts (low melting point substances such as wax) are difficult to handle at the time of formulation. However, capsule filling and direct tableting with such substances can be much easier by adsorbing them to Neusilin UFL2 and US2.
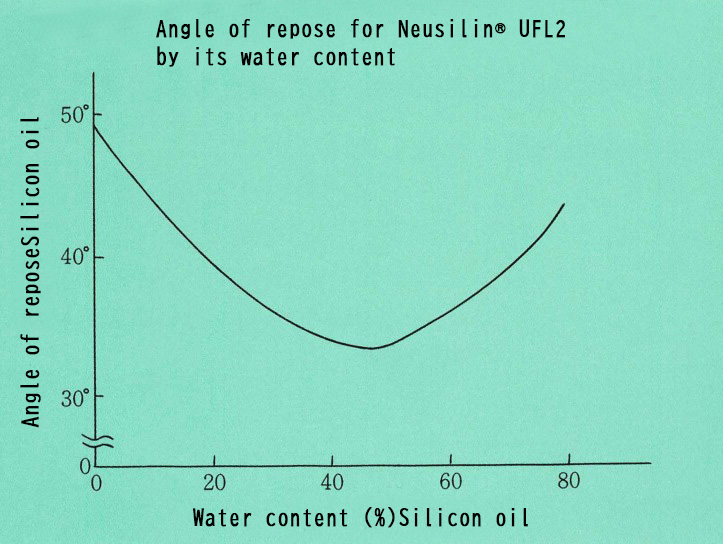
Angle of repose for Neusilin® UFL2 by its water content
Neusilin UFL2 and US2 still maintain their powder state even after they adsorb a liquid equivalent of 200 to 250% of their own weight. The adsorptive capacity of Neusilin UFL2 and US2 is due to its extremely large specific surface area and porous reticulated structure. The obtained powder does not exude oil and has excellent fluidity and compressiblity. It is very effective as a powder for filling capsules.
Powderization of Egg yolk lecithin
Adsorbing 1 part of egg yolk lecithin to 1 part of Neucillin UFL2 creates powder with excellent fluidity
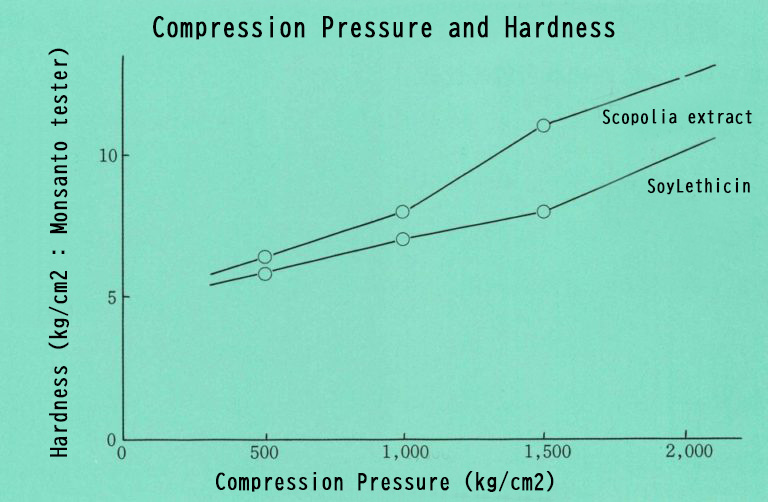
Powderization of extracts and oils
Extract and oil adsorbed Neusilin® UFL2 (50%) is prepared, and the same amount of lactose is added and mixed. When the mixed powder is statically compressed, a robust tablet is created with no sticking on punches or dies. In addition, no exudation of extracts or oil is observed.
Scopolia extract: 25%
Lactose powder: 50%
Neusilin® UFL2: 25%
Soy lecithin: 25%
Lactose powder: 50%
Neusilin® UFL2: 25%
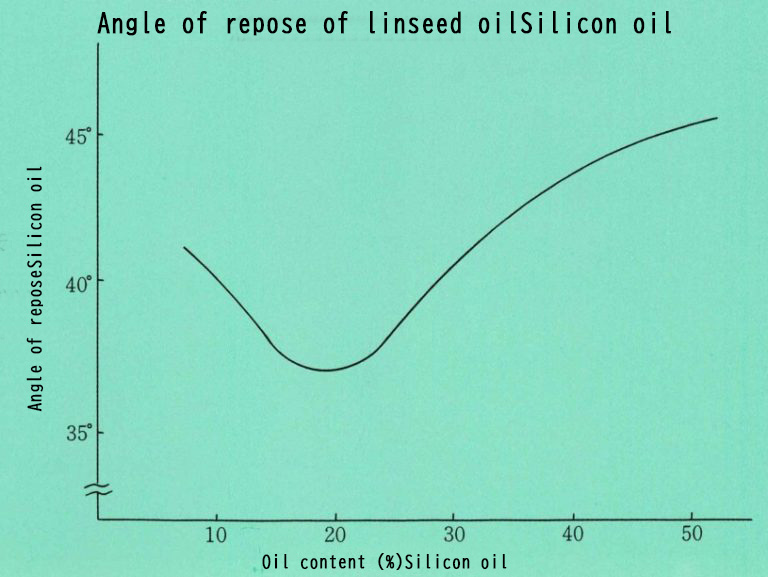
The figure below shows the angle of repose of Neuslin® with adsorbed boiled linseed oil.
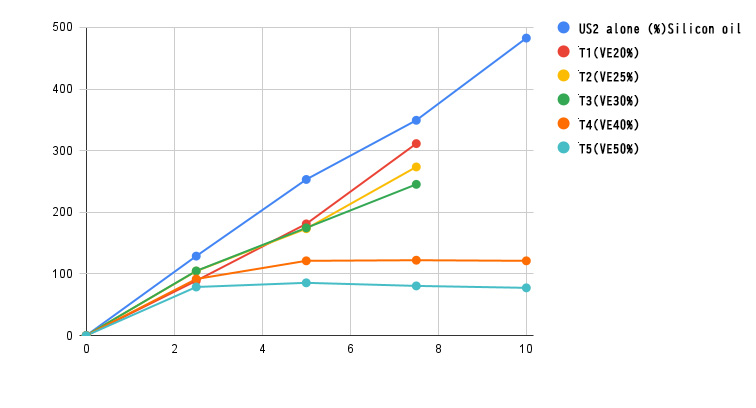
Adsorption of tocopherol acetate (VE) with Neusilin US2
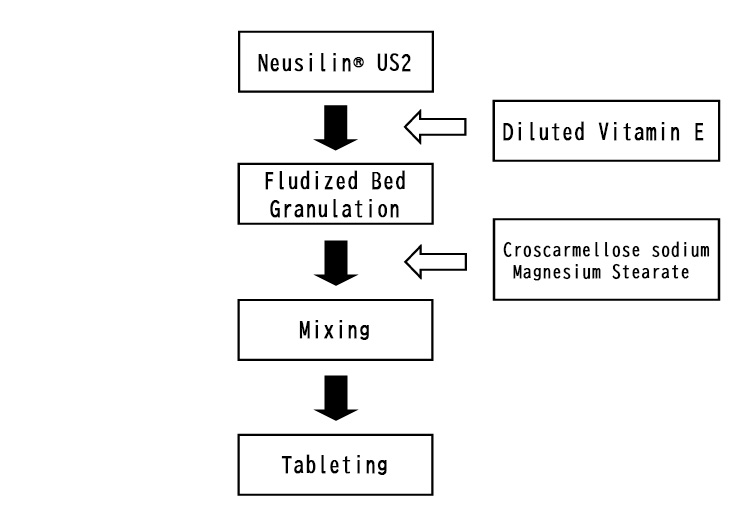
We will introduce tablets containing tocopherol acetate as oil adsorption.
Neusilin US2 is placed in a fluidized bed granulator to adsorb a predetermined amount of tocopherol acetate diluted with ethanol, which is sprayed onto Neusilin inside. After drying out the ethanol, croscarmellose sodium and magnesium stearate was mixed. The mixed powder was compressed with a single-punch tableting machine.
Formulation
| Composition | T-1 | T-2 | T-3 | T-4 | T-5 |
| VE(%) | 20 | 25 | 30 | 40 | 50 |
| Neusilin ®US2(%) | 76 | 71 | 66 | 56 | 46 |
| Croscarmellose sodium | 3 | 3 | 3 | 3 | 3 |
| Mg-St | 1 | 1 | 1 | 1 | 1 |
| Neusilin US2(%) | 26.3 | 35.2 | 45.5 | 71.4 | 108.7 |
Evaluation of compression pressure and tablet hardness after oil absorption
With tablets containing 20-30% tocopherol acetate, the hardness increased proportionally with compression pressure, and the increase was almost the same as that of US2 alone. Even with a molding pressure of 500 kg, the tablet hardness was maintained at 150 kg / cm2, and no exudation of tocopherol acetate was observed.
On the other hand, in tablets containing 40-50% tocopherol acetate, the tablet hardness did not increase as the compression pressure increased, and tocopherol acetate exudation was observed. The evaluation was carried out with 300mg tablet.